
Worldwide Energy
Gas hydrates are the most abundant energy source in the world,
exceeding the combined energy from all oil, coal, and
natural gas reserves, with less harmful emissions.
Natural gas hydrates are granular, ice-like solids containing natural gas nested within a lattice of frozen water. They are also called methane hydrates since the most common gas found in them is methane, the main component of natural gas.
(photo: courtesy of NOAA OKENOS exolorer program)

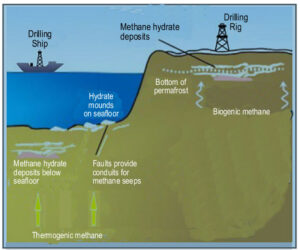
Gas hydrates are found in high-pressure, low-temperature environments, like in sediments beneath deep seafloors and frozen ground in arctic regions.
Natural gas in the form of methane produced from globally abundant gas hydrate deposits is the world’s biggest source of cleaner energy that produces lesser emissions.
Oceanic gas hydrate occurs in vast volumes in shallow sediments along deep-water continental slopes, where the continental plates meet deep-sea regions.
They occur naturally at depths of 500 to 2,500 meters, characterized by high pressures of about 2,000 psig (pounds per square inch) and low temperature of about 40°F (4°C).
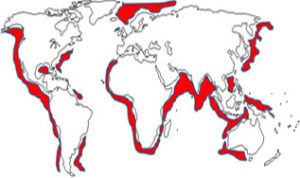
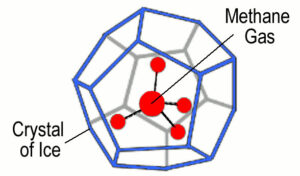
Since the water and gas molecules in hydrate crystals are held together by weak forces, they can be easily dissociated to produce methane gas.
When gas hydrate is either warmed or depressurized, one volume of gas hydrate releases 164 volumes of methane and 0.8 volume of fresh water.
Technically recoverable global gas reserves are estimated at 300 trillion cubic meters (10,600 trillion cu ft) with a market value ranging from US$ 30 to 150 trillion. Only a 10% recovery from this gigantic reserve can meet escalating global energy demands for several hundred years.
